Rejoinder: More Limitations of Bayesian Leave-One-Out Cross-Validation

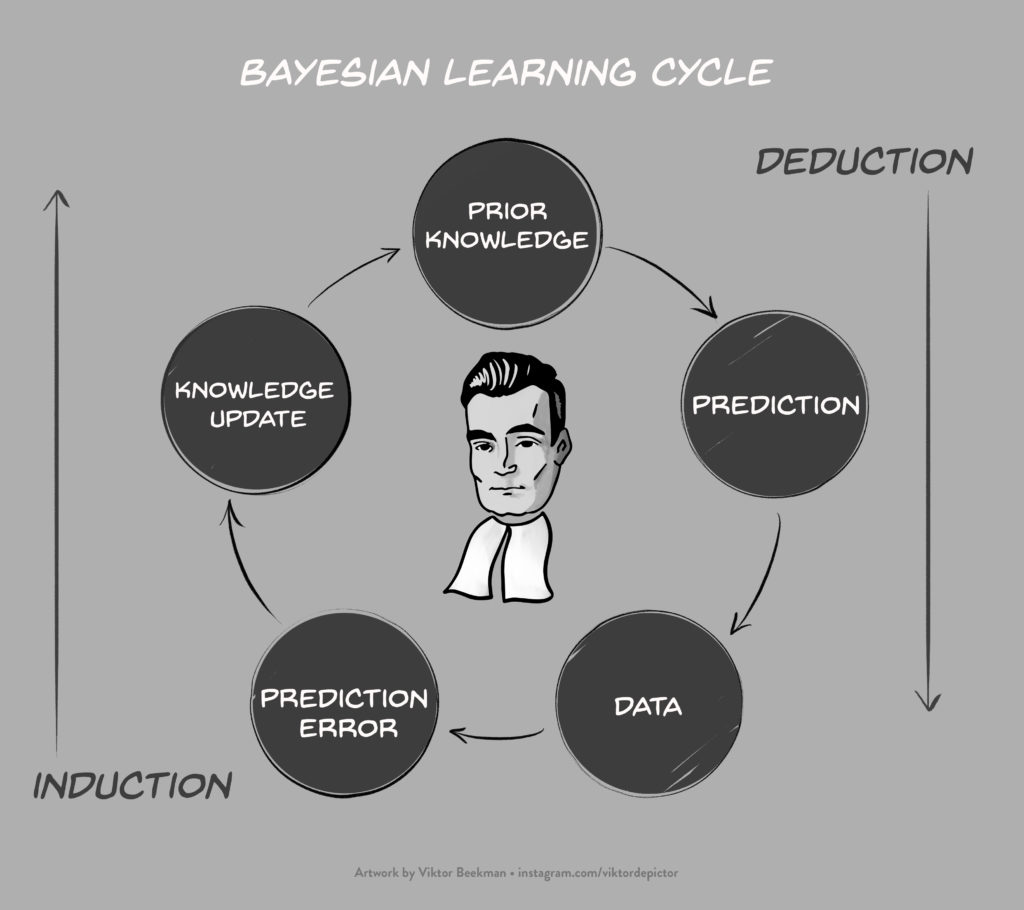
In a recent article for Computational Brain & Behavior, we discussed several limitations of Bayesian leave-one-out cross-validation (LOO) for model selection. Our contribution attracted three thought-provoking commentaries by (1) Vehtari, Simpson, Yao, and Gelman, (2) Navarro, and (3) Shiffrin and Chandramouli. We just submitted a rejoinder in which we address each of the commentaries and identify several additional limitations of…
read more